About Formycon
Commercial Stage Biosimilar Developer
Through our biosimilar development projects, Formycon creates not only highly effective, more affordable biologic drugs for patients but also long-term value for its investors. Biosimilars are a new class of medicines with a very promising future, and our product candidates are targeted at multi-billion-dollar markets.

Equity Story
Focus on What Matters Most – Our Biosimilar Platform
Formycon is an established, successful, and independent pure-play biosimilar company specializing in the development of cost-effective follow-on products for complex biopharmaceutical medicines. In the rapidly growing biosimilar market, we are committed to expanding and advancing our scalable biosimilar platform. Currently, this platform includes three approved biosimilars for ophthalmology and immunology indications, providing millions of patients worldwide with access to essential and affordable therapies. Additionally, we are developing four next-generation biosimilar candidates, with more to follow.
Expertise, Agility, and Flexibility as the Recipe for Success
As a pure-play biosimilar company, Formycon holds a distinct competitive advantage. Our focused strategy, agile development processes, and strong partnerships allow us to efficiently develop high-quality follow-on products and quickly adapt to market changes. This flexibility, combined with our high level of scientific expertise, positions us as a reliable and preferred partner in the highly regulated biopharma market. Our goal is to meet the growing global demand for biosimilars and ensure long-term value creation through a diversified portfolio and strong partnerships.
Strong Partnerships for Economic Success
Strategic partnerships with established manufacturing and commercialization partners are critical to the economic success of our biosimilars. In manufacturing, we select the most suitable partner for each of our biosimilars to ensure a cost-efficient and competitive production process. For global distribution, we form commercialization partnerships with leading pharmaceutical companies such as Fresenius Kabi, Sandoz, and Teva. Thanks to this hybrid business model, we have laid a solid foundation for continuous revenue streams and further growth.
Participate in Growth with Us as Biosimilar Market Specialists
For investors, Formycon’s pure-play approach offers an attractive opportunity to benefit from the dynamic biosimilar market. We develop products that meet the needs of various stakeholders. In addition to patients, doctors, and healthcare systems that directly benefit from the use of biosimilars, pharmaceutical companies also seek our biosimilars to expand their product portfolios in distribution.
With our recent uplisting to the Prime Standard of the Frankfurt Stock Exchange, Formycon has gained significance in the international capital markets. Our solid financial structure and experienced management team provide a reliable foundation for further growth and a strong market position in key global markets.
FAQ
In three to five years, we envision Formycon with at least three or four biosimilar products distributed internationally through commercialization partners. Beyond that, our product pipeline will have matured further and expanded. The company will have established itself as a globally renowned player in biosimilars, grounded in solid financials.
Unlike generics, which are chemically synthesized and contain the exact same active ingredient as the originally patented drug, biosimilars are produced using living organisms. This is usually done in cell cultures, such as bacteria, yeast cells, or mammalian cells1. This is why biosimilar development takes between 7 to 10 years2 (compared to 2 to 3 years for generics3) and requires a budget of between 150 to 300 million euros3 (compared to 5 to 10 million euros for a generic3).
Sources:
1 What are biosimilars?
2 IQVIA Assessing the Biosimilar Void.
3 Company estimates; development times and costs vary greatly
depending on the indication and type of active ingredient.
Our most valuable assets are our pipeline projects, as they create value. Therefore, our focus will remain on expanding our platform. Currently, we plan to launch a new biosimilar candidate every 12 to 18 months.
We evaluate candidates independently of their therapeutic area. In our view, the key to a sustainable and value-generating platform is a smart mix of niche and blockbuster products in selecting the right candidates.
The likelihood of a biosimilar receiving approval is around 95%, provided that development has been conducted with precision and care throughout. The decisive inflection point is known as the “Technical Proof of Similarity” (TPOS). From this stage onward, the probability of successful approval increases with each phase.
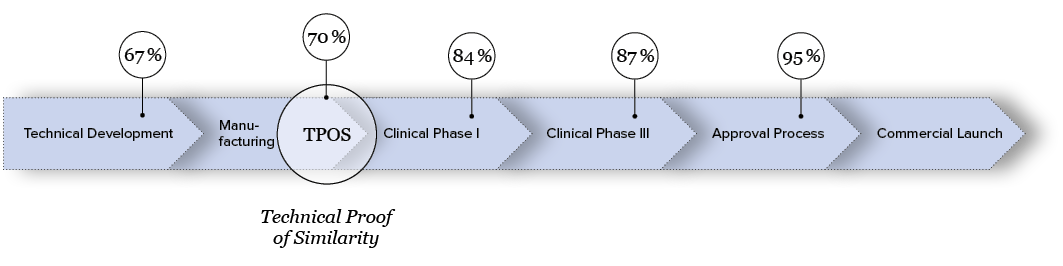
Source: “The Path Towards a Tailored Clinical Biosimilar Development,” Schiestl et al., 2020
Formycon uses AI tools to speed up and streamline internal processes. AI can also be used in product development, particularly for the efficient selection of excipients in formulation development and for optimizing formulation stability. Additionally, we use AI to generate better forecasts from our data. In the future, AI will undoubtedly play an even larger role in diagnostics, both within clinical trials and in clinical practice.
Compared to large pharmaceutical companies, we are able to operate like an agile speedboat, adapting quickly and flexibly to current conditions. In the biosimilar field, it’s unique that competitors can also be potential partners. What sets Formycon apart are our experts exclusively focused on biosimilars, with many years of experience in the development and approval of biosimilars, and who thoroughly understand all opportunities and challenges across the value chain. Furthermore, there are over 200 biological active ingredients, and no company, not even large pharmaceutical companies, can develop all of them independently. This makes us an attractive partner for them as well.
The development of this class of drugs clearly points to further uptake and establishment of biosimilars. This is evidenced by the cost savings that biosimilars have achieved in recent years. IQVIA’s Global Use of Medicines report projects that biosimilars will generate global savings of $290 billion by 2027. These savings provide healthcare systems with more financial flexibility in other areas, making biosimilars an essential contribution to sustainable healthcare.
FYB201 is distributed under the brand name Ongavia® in the UK, Ranopto® in Canada, and Ranivisio® in Europe and other countries by Teva Pharmaceuticals Ltd. In the U.S., Cimerli® is marketed by Sandoz, and in the MENA region, MS Pharma markets it under the brand names Uptera® and Ravegza®.
For FYB202/Otulfi®, our commercialization partner for key global markets is Fresenius Kabi.
For FYB203 and other biosimilar candidates, no commercialization partnerships have been announced yet.
We see ourselves as an R&D powerhouse with a fully scalable biosimilar development platform and are very comfortable in this role. However, Formycon is growth-oriented and aims to establish itself as a global player in biosimilars. This fundamentally includes evaluating opportunities for inorganic growth along the value chain.
Formycon is a growth-oriented company with currently only one product on the market. Therefore, to support sustainable and value-generating company development, we are likely to reinvest returns into existing and new development projects, and we do not currently plan to pay a dividend.

